Economic Justice Discussion
IGJ – Nov 2020
Reporter(s):
Agung Prakoso, Intan Baretta Nur Azizah, Rachmi Hertanti
Introduction
A belief that the only way to return to normal life is to ensure that the spread of the virus can be slowed down while accelerate research and development of diagnostic technology, availability of adequate medical tools and treatments, and including vaccines. However, in the process, there are still many countries that do not yet have the technology and infrastructure which are capable of procuring vaccines, especially the knowledge about this is often monopolized by corporations from developed countries. Indonesia itself experiences limitations in infrastructure and technology, thus vaccine procurement still requires cooperation with other countries through bilateral and multilateral diplomacy.
The business approach is still applied in handling Covid-19. Strengthening patent protection regulations which impact on the monopoly of knowledge and production leads to price and supply controls. This will certainly make developing countries, especially Indonesia, face the problem of financing the procurement of vaccines and other medical devices related to the COVID-19 pandemic, especially in the midst of an economic recession that is still looming.
Implementation in vaccine production and distribution has become the spotlight of the people nowadays, especially to ensure access to vaccines and other medical devices as public goods for all levels of the society without exception. Including the vaccines in circulation, their safety and effectiveness will be guaranteed for the people.
This issue has been discussed by Indonesia for Global Justice (IGJ) together with the Indonesia AIDS Coalition (IAC) and the Coalition for Affordable Medicines in an online discussion entitled “Diplomacy of Indonesian Covid-19 Vaccines” on 12 November 2020. Various sources and perspectives have been drawn from CSOs. , academics, public health experts, the government, and the House of Representatives of Republic Indonesia. This discussion aims to ensure that the public is informed about the importance of accessing vaccines and medical devices into public goods and not being commercialized in the midst of a pandemic, including ensuring the protection of IPR, especially patents, is not misused in the interests of pharmaceutical companies and groups trying to make a profit amid the current pandemic.
The Global Effort in Granting Vaccine Access for All
“No one is safe until everyone is safe.”
(Dr.Tedros Adhanom Ghebreyesus)
Speaking of the handling of covid-19, almost everything is related to the issue of protecting Intellectual Property Rights (IPR). Third World Network researcher, Lutyah Hanim, explained that almost all health products in handling Covid-19 such as test kits, diagnostics, masks, medicines, vaccines and ventilators are protected under patents, trade secrets and industrial designs. Moreover, the TRIPS rules at the WTO are still being maintained until now, and Indonesia has changed its IPR rules a lot after ratifying the WTO.
IPR protection provisions have monopolized knowledge which is fully controlled by the pharmaceutical industry in developed countries. Therefore, handling Covid-19 poses enormous challenges, especially when pharmaceutical companies still apply the business as usual approach in practice. We are facing the Pandemic, hence it is very important to ensure that everyone in the world has access to it and that no one is left behind.
In handling the Covid-19 pandemic, there are many challenges, both in terms of economic and security, access (price, accuracy, adequacy), allocation, and implementation (distribution). With a business approach by corporations, it definitely will be very difficult for us to be able to answer the challenges, especially if IPR protection regulations are still tightened. Furthermore, Hanim explained that at the international level, there were at least several efforts made by countries in the world to answer the challenges of handling the Covid-19 pandemic.
First, bilateral efforts. There have been many countries that have conducted bilateral transactions with pharmaceutical companies, especially developed countries which have the ability to buy directly. However, most certainty, with bilateral efforts or what is often referred to as the issue of vaccine nationalism, it tends to raise the problem of disparities with developing countries and least developing countries due to their limited financial capacity. This effort is implemented even though the vaccine has not been declared safe (See Figure 1). Today, we often hear one-sided claims from pharmaceutical companies competing to say that the vaccine they invented has up to 90% effectiveness. For instance, of the 154 pre-clinical vaccine candidates currently being tested, only 21 vaccines are entering phase 1 clinical trials that are being tested for safety in healthy young people, 13 vaccines are currently being tested in phase 2 clinical trials in groups of people, and so far there are only 10 vaccine candidates that are already in phase 3 clinical trials and are currently undergoing international Covid-19 impact trials. The final results are yet to be announced. Therefore, there is no vaccine that has been approved and given permission by WHO for the public.
Second, the TRIPS Waiver Effort. This proposal was proposed on 2 October 2020 by India and South Africa at the WTO to allow all countries not to provide or not enforce IPR regulations related to handling Covid-19 both for medicines, test kits, vaccines, or other technologies during the pandemic until global immunity is reached. This is highly important in order to provide ample policy space for all countries, especially developing countries, for research, local manufacturing, supply, and others in the context of handling Covid-19. Indonesia itself has agreed, but in the process of discussion there is still a great tug of war between supporting and non-supportive countries. Although Indonesia supports the TRIPS Waiver proposal, the Ministry of Foreign Affairs itself will still observe to what extent the dynamics of the TRIPS Waiver proposal will provide Waiver as a whole or whether there will be more bargains in the future. In this case, Indonesia’s position is more to find a middle point, because, if it is too extreme, it will also face political challenges.
Responding to this, Sriwijaya University International Relations Scholar, Ferdiansyah, said that the diplomacy conducted by the government towards vaccines should emphasize humanitarian diplomacy. The South-south cooperation model can also be encouraged, because in general this cooperation model builds solidarity, rather than competition brought by northern countries. Therefore, the ideal choice for a country like Indonesia is if the TRIPs Waiver proposal can be agreed upon, hence we can have a policy by producing local vaccines. If it can be accepted and implemented in its member countries, TRIPs Waiver will be able to overcome IPR barriers to ensure fair distribution, policy regulation, including efforts to reduce prices.
Third, efforts through Access to Covid-19 Tools (ACT) Accelerator. This initiative is a global collaboration to accelerate the development of production and equitable access to Covid test kits, treatments, and vaccines. There are several parties involved, including WHO, Gavi, CEF, Unitaid, Bill & Melinda Gates Foundation, Wellcome, World Bank, The Global Fund, and Find. Especially for vaccines, it is discussed in one pillar called The Covid-19 Technology Access Poll (CTAP). CTAP as a forum for sharing voluntarily shares knowledge, intellectual property, and data related to COVID-19 health technology. However, this is the challenge of CTAP, where it is only voluntary where many pharmaceutical companies are reluctant to share, especially with the profitable approach. Other challenges that also arise in this initiative are related to transparency and questionable governance, particularly in making vaccines and other medical devices as something that public may know (or consumable).
Figure 1
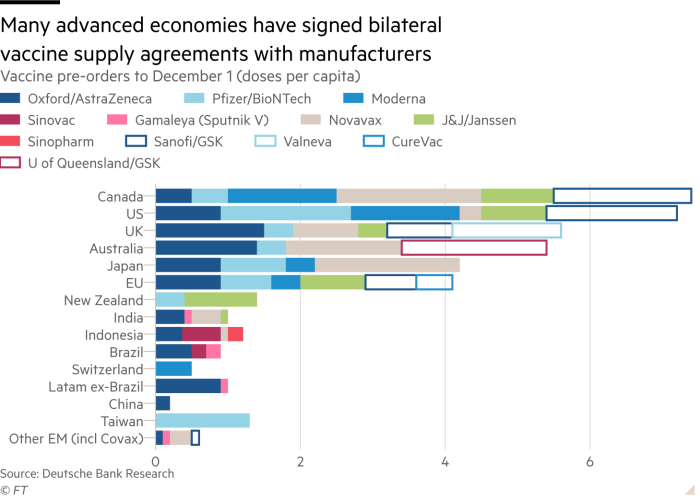
Vaccine Diplomacy: “Efforts to Procure Vaccines in Indonesia”
Indonesia is currently very active in conducting diplomacy and negotiations to get access to vaccines. Erik Mangajaya, Directorate of Law and Economic Agreements at the Ministry of Foreign Affairs of the Republic of Indonesia said that efforts to procure vaccines by the Indonesian government are conducted in two ways, namely bilateral agreements, CEPI (Coalition for Epodemic Preparedness Innovation) and GAVI (Global Alliance for Vaccines and Immunization) cooperation, and national vaccine development efforts, known as red-and-white vaccines. Vaccine diplomacy implemented by the Government is definitely in order to ensure the availability of vaccine needs for the people of Indonesian.
Through Presidential Decree No. 18 of 2020 Articles 9 and 10 concerning the National Team for the Acceleration of Handling COVID-19 and Regulation in Lieu of Law No. 1 of 2020, the Minister of Foreign Affairs plays a role in the COVID-19 Vaccine Development Team. Apart from that, Presidential Regulation No. 99 of 2020 has also mentioned cooperation with international institutions or bodies such as CEPI and GAVI. This legal basis then becomes the basis for the implementation of vaccine diplomacy to accelerate and ensure the procurement of the Covid-19 vaccine according to government needs.
Up to now, bilaterally, Indonesia has collaborated with several Covid-19 vaccine candidates, including with the People’s Republic of China through Sinovac, England through Astra-Zeneca, United Arab Emirates through G24. On 3 December 3 2020, through the Decree of the Minister of Health of the Republic of Indonesia number HK.01.07/MENKES/9860/2020 concerning the Determination of Vaccines for the Implementation of Covid-19 Vaccination, the Government has determined six types of vaccines to be applied in Indonesia. First, the vaccine produced by PT Bio Farma (Persero). Second, the vaccine produced by AstraZeneca. Third, the China National Pharmaceutical Group Corporation (Sinopharm) vaccine. Fourth, Moderna. Fifth, Pzer Inc. and BioNTech. Sixth, Sinovac Biotech.
In seeking vaccines, the Government of Indonesia also encourages cooperation related to technology transfer. For example, the cooperation that the government has built with Sinovac also requires the transfer of technology to the national industry. According to Erik, this is highly important, especially when the Covid-19 occurs, it has had a significant impact on the national health sector which is greatly dependent on imports. Not only medicine, but also other medical devices. For this reason, the Government of Indonesia will take advantage of this situation to encourage increased independence for the Indonesian health industry. For instance, one matter which the Government is also targeting in the CEPI collaboration is exploring opportunities for cooperation as a CEPI vaccine development and production partner where Bio Farma has been included in the shortlist of potential manufacturers for CEPI’s Covid-19 vaccine.
Figure 2

Responding to this, Member of Commission IX of the Indonesian House of Representatives, Netty Prasetiyani, also expressed Commission IX’s insistence on the Government to make Covid-19 a momentum for Indonesia to be able to implement Presidential Instruction No.6 of 2016 concerning accelerating the development of the pharmaceutical industry and medical devices. Ensuring national independence and the level of domestic content can be improved. For this reason, in relation to the procurement of vaccines, Commission IX hopes that Indonesia can immediately produce the “Red-and-White” Vaccine, although it may only be completed in 2022.
Another thing that was emphasized by Netty was regarding the procurement of vaccines, she stressed the importance of the government in conducting good public communication. This is due to many people are distorted from information which has an impact on the skeptical response of the efforts being made by the Government today. Don’t let this become a major obstacle in handling Covid-19.
Information disclosure to the public that must be implemented by the Government, namely: First, regarding the safety assurance of vaccines. There needs to be a sufficiently detailed explanation from the government regarding the effectiveness and safety of vaccines. The government must ensure that the vaccines purchased can be guaranteed not to cause harmful side effects, thus the public is willing to be vaccinated. Based on the current information available, the Government claims the third phase of the clinical trial of the Sinovac Vaccine can be said as safe, because no harm has happened to the volunteers who received the vaccination. Side effects were found but were in minor scale and mild level in some of the volunteers.
Second, about the ability to procure vaccines. To achieve herd immunity, at least the government must vaccinate 70% of the total population, and that must be conducted twice. Based on Presidential Decree No. 99 of 2020 Presidential Regulation on Vaccines Procurement and Implementation of Vaccinations, the government has appointed BioFarma directly. BioFarma’s production capacity is of course also limited and must partner with other companies to expand their production capacity. Regarding vaccine availability, on 6 December 2020 the Government announced that as many as 1.2 million doses of Sinovac Vaccine had arrived in Jakarta, and another 1.8 million doses would be sent in January 2021.
Third, the need for transparency in the aspect of financing. The price issue has become a sensitive issue, for example the past experience of the Rapid test and PCR pricing. Including the disclosure of information regarding the ability of the state budget to provide vaccines for the entire population for free. The latest decision from President Jokowi on 16 December 2020, he stated that the Government will make vaccines free for all people.
However, transparency regarding prices obtained from pharmaceutical companies also needs to be disclosed, because this will have an impact on the burden on the state budget. For example, in the discussion of the Committee on Medicine Management at Commission IX also noted the amount that must be paid by the Government regarding the purchase of raw materials from Sinovac, which could reach tens of trillions of rupiah. The process must be transparent, the budget used is also accountable. The Government must be able to explain how much is the purchase amount, how much is the price, then when it is mass produced, how much will it cost per unit of vaccine.
Vaccination, Who Will Be Served First?
In order to achieve “herd immunity” and reduce the death rate of Covid-19, vaccination is an utmost important step. The Expert Council of the Indonesian Public Health Expert Association, Dr. Sumarjati Arjoso, said that vaccination is an effective and essential way to increase immunity and prevent disease transmission. However, vaccination must be conducted carefully, because it must ensure its safety and effectiveness/usefullness.
The Ministry of Health has made a Grand Design for the implementation of the Covid-19 vaccination. At least vaccination is conducted for 80% of the total population. In fact, the government has determined priority groups of vaccination activities (See Figure 3). Regarding the priority criteria for vaccine recipients, the government must explain openly, including the priority areas for vaccine recipients. This is a massive problem as well, because the readiness of healthcare services in Indonesia is different at every district/city.
Sumarjati further explained that the Minister for National Development Planning Agency/Head of National Development Planning Agency herself estimated the budget requirement for the procurement of Covid-19 vaccines, which reached IDR 46 trillion to IDR 62 trillion, this does not include the budget for providing facilities and infrastructure for immunization services. Moreover, logistic and distribution support is also a problem in itself. Therefore, the State must be able to communicate to everyone about the guarantee that there will be no discrimination against access to vaccines, both in terms of price, distribution, and affordability.
COVID-19 VACCINATION TARGETS
Priority Phase based on vaccine availability, population and risky district,
stages of use and index of use
IDEAL: THE WHOLE POPULATION
OPTIMUM: 80% of POPULATION AT RISK OF INFECTION

IGJ Contact
Email: keadilan.global@gmail.com / igj@igj.or.id
Website: www.igj.or.id
This discussion presented Lutyah Hanim from the Third World Network, Erik Mangajaya from the Directorate of Legal and Economic Agreements at the Ministry of Foreign Affairs, Dr. Sumayati Arjoso from the Association of Indonesian Public Health Experts, Netty Prasetyani, Member of the House of Representatives Commission IX, and Ferdiansyah, Scholar of International Relations, Sriwijaya University.
https://www.gavi.org/tag/covid19
This article has been published on Katadata.co.id entitled “Terawan Tetapkan 6 Jenis Vaksin Covid-19 yang Akan Digunakan di RI”, https://katadata.co.id/febrinaiskana/berita/5fcbb6ba0f184/terawan-tetapkan-6-jenis-vaksincovid-19-yang-akan-digunakan-di-ri
This article has been published on Kompas.com entitled “Mengenal Vaksin Sinovac yang Telah Tiba di Indonesia”, Click to read: https://www.kompas.com/tren/read/2020/12/08/110300765/mengenal-vaksin-sinovac-yang-telah-tibadi-indonesia?page=all
